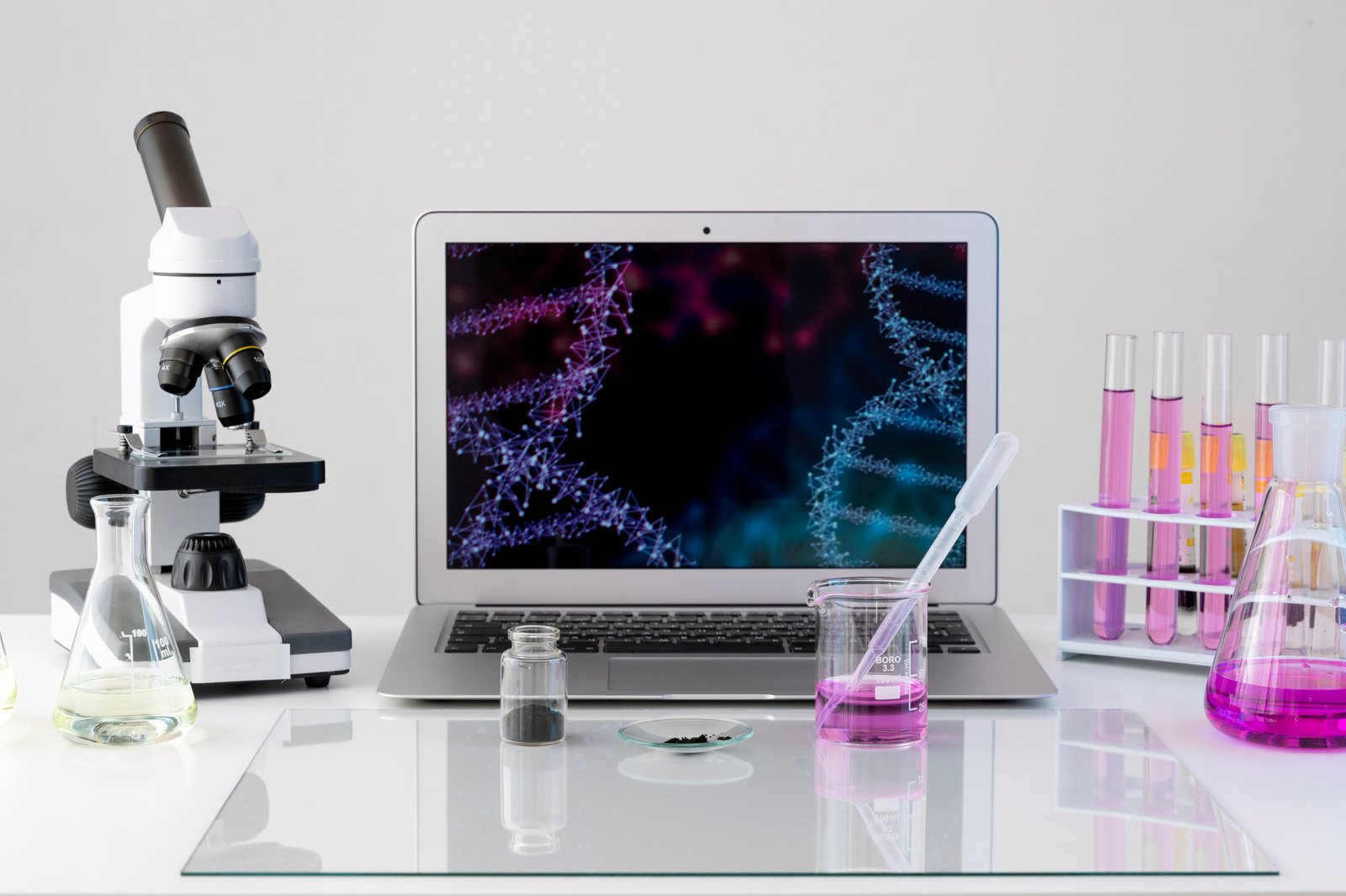
Experiment with the best lab products
Oasis infotech is ISO 9001:2015 certified, a leading application development company, with many industry specific products in QC/QA area as core competence, offering LIMS (Labratory Information Management System), QMS (Quality Management Systems) and Automation Solutions in making laboratories more productive and efficient with integrated informatics, services and solutions.
Oasis products line provide state-of-the art technology and consulting services for automating modern laboratories and has been instrumental in developing a wide range of associated applications to computerize manufacturing units / analytical and research labs and other related activities.
LIMS Solutions Across Industries
Scalable and compliant systems tailored for diverse laboratory needs.
Pharmaceuticals and API Manufacturing
Learn more about our solutions for Pharmaceuticals and API Manufacturing
Chemicals, Agriculture Chemicals, Oil/Gas, Petrochemicals
Learn more about our solutions for Chemicals, Agriculture Chemicals, Oil/Gas, Petrochemicals

Environment and Water Laboratory
Learn more about our solutions for Environment and Water Laboratory
0
Labs Served
0
Tests per Year
0
Industries
0
Clients Worldwide
Industries We Serve
Pharmaceuticals
Ensuring quality and compliance in drug testing and manufacturing.
Food & Beverages
Supporting safety and quality checks in food production.
Water Testing
Providing accurate analysis for water safety and compliance.
Medical Devices
Ensuring compliance and reliability in device manufacturing.
Resources/News & Updates
RESOURCES
Why Choose Us
Delivering trust, security, and seamless integration for your laboratory needs.

Data Security & Compliance
We ensure maximum data protection and follow global standards like 21 CFR Part 11 and WHO-GMP.
Integration Capabilities
Our solutions integrate seamlessly with SAP, ERP systems, and laboratory instruments.
Implementation & Support
From implementation to support and training, our experts ensure smooth adoption.
Screenshots & Demo Tour
Explore our platform with sample screens and a quick walkthrough video.
Watch this 2-minute demo tour to explore how our platform streamlines your laboratory processes.
Innovative software Solutions
Client Testimonials
Hear from our valued clients and explore their success stories.
Why Oasis
Domain Expertise
One of the group companies having analytical labs, R&D centers, formulation units, itself serves as a strong guide and senior resource team of QA, QC experts and IT experts in-house to deliver solutions with current amendments in pharmacopoeias / D&C Act and the Rules.
Global Experiance
Oasis has given a new dimension to implementation of LIMS in pharma sector keeping in view not only Indian regulations but Global regulations as well as best practices inline with GMP guidelines.
Integrated Solution
Oasis unique platform offers integrated LIMS, ERP, QMS, Instruments as a single product to support pharmaceutical value chain – from production line optimization, through to quality control, quality assurance and batch release with validation master plan and execution.
Largest clientele
Oasis has largest clientele and presence in various segments MNCs, SMEs, Govt., PSUs, in Pharma manufacturing, API/Bulk drug industries, R&D Laboratories, Commercial / public Test Laboratories, Industrial QA/QC laboratories, Chemicals, Petro etc.
Careers / Work with Us
Oasis Infotech is ISO 9001:2015 certified — innovating in LIMS, QMS, and lab automation with compliance-driven solutions.
Our Culture & Mission
We drive innovation and deliver reliable, compliant informatics services globally. Join us to help labs become more productive and efficient.
Current Openings
Compliance & Certifications
We comply with global standards to ensure quality, safety, and data security.
ISO Certified
International quality management standards ensuring best practices.
NABL Accreditation
Recognized for competence in testing and calibration labs.
FDA Compliance
Adheres to US FDA regulations for electronic records & signatures.
WHO-GMP Standards
Follows WHO guidelines for manufacturing & quality assurance.
GDPR Compliance
Protecting customer data with strict privacy and security measures.
Data Security Practices
Encryption, access control, and monitoring to safeguard data.
Our Customers